Emergo Group Medical Device Regulatory
A valuable reference tool for medical device Regulatory Affairs (RA) and Quality Assurance (QA) professionals. Quick access to global medical device regulations, daily medical device regulatory updates, contact information for 60+ Ministries of Health and Certification Bodies. Emergos mobile app contains valuable reference information for regulatory and quality assurance professionals. Heres what you will get:
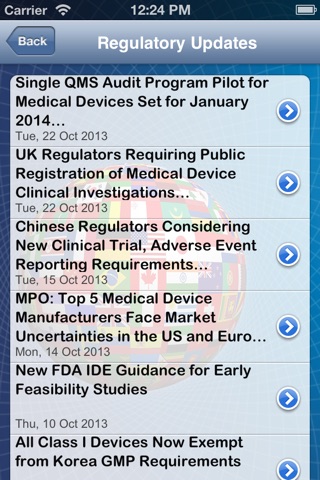
• Latest regulatory updates from the Emergo blog
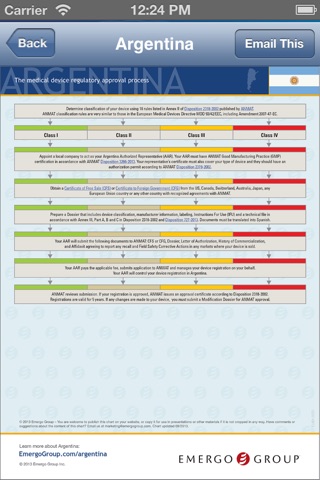
• Quick access to device regulations for 20+ countries
• Downloadable flow charts, white papers plus videos
Plus, the app contains fully interactive copies of the following regulations, allowing you to quickly jump to relevant sections:
• US FDA 21 CFR Part 820 (Also includes parts 801, 803, 806, 807, 809, 812, 821, 822, 860)
• EU MDD 93/42/EEC (Complete with all sections linked for easy access, plus 2007/47/EC)
• EU IVD Directive (98/79/EC)
• EU DIRECTIVE 2007/47/EC
This app includes regulatory information covering 90% of the global medical device market including:
• Australia
• Brazil
• Canada
• China
• Europe
• Hong Kong
• India
• Japan
• Malaysia
• Mexico
• Russia
• South Korea
• United States